-40%
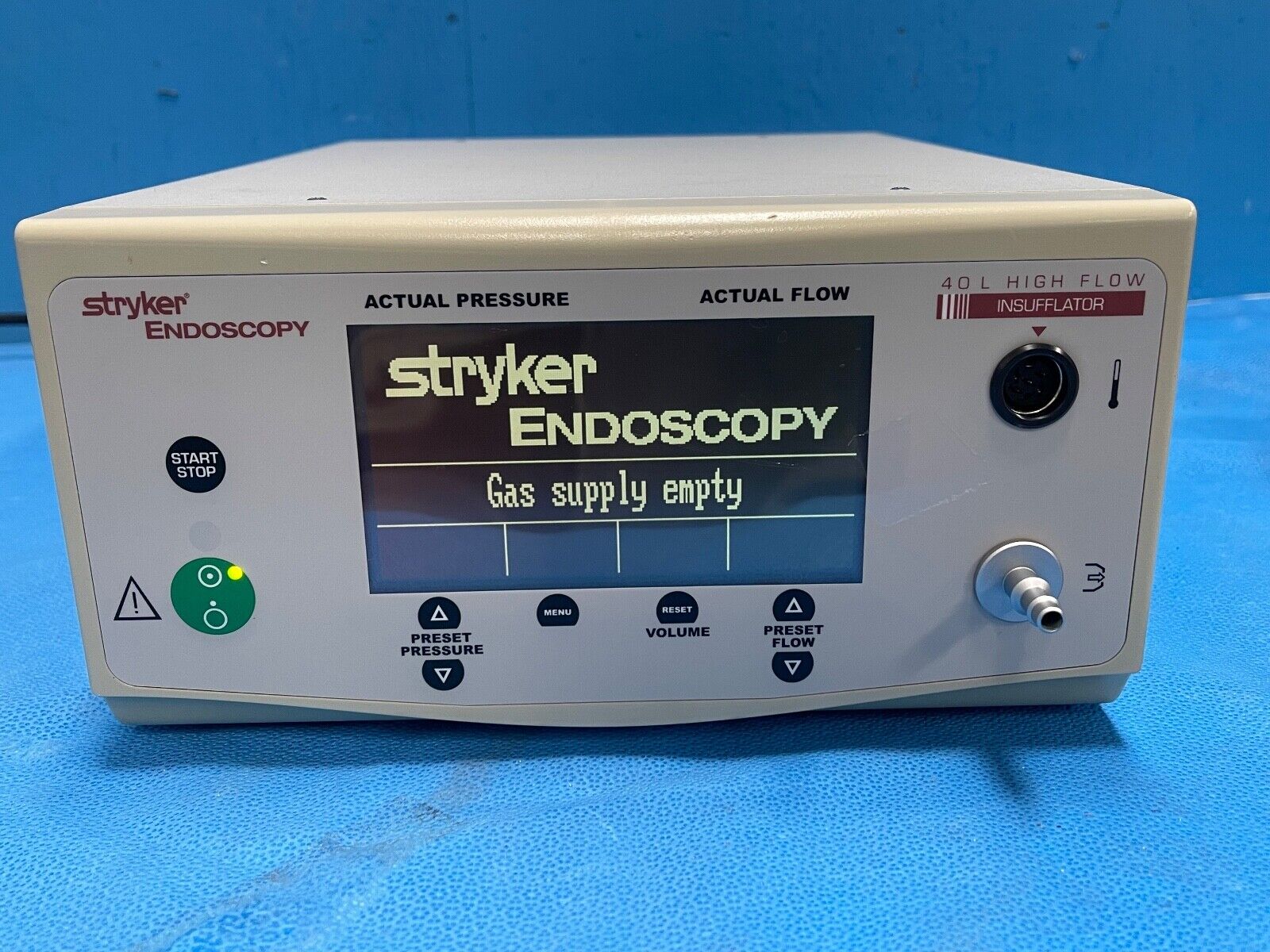
Stryker 40L High Flow Insufflator 0620-040-000 / F105 - missing back gas connect
$ 171.6
- Description
- Size Guide
Description
1 - Stryker 40L High Flow Insufflator 0620-040-000 / F105 ....Powers on and goes through self check.
Recognizes that there is no gas supply.
Buttons work great and allow to circulate through menus.
Display and front membrane are all in excellent condition.
Case and Warning labels on top are in very good condition but do have stickers, scuffs, scratches, dirt, etc.
** This unit is MISSING the main gas supply line connector on the back bottom left-hand corner.
** See close up photo.
Not sure if the hospital robbed from this unit in order to repair another one. Looks like the unit was possibly dropped and that connector hit first and pushed back panel inward ever so slightly. Possibly connector was damaged and they removed it and they never bothered to order a new part and repair it. This unit was purchased along with three other insufflators so the facility most probably bought new equipment to replace these older insufflators.
Disclaimer
: This item is sold as is, no warranties implied. The item described and/or pictured offered here is in no way certified for, recommended for, or offered for any specific use. The purchaser agrees that the seller shall not be held responsible or liable for any injuries or damages, whether incidental or coincidental, associated in any way with this equipment. It is the sole responsibility of the purchaser to obtain qualified help to ensure the proper functioning, calibration, and safety (mechanical, electrical, etc.) of this equipment. The purchaser by biding on this item, indicates their knowledge of an agreement to the terms of this disclaimer. This item is sold completely as is/ as shown with no warranty either expressed or implied. The sale of this item maybe subject to regulations by the U.S. Food and Drug Administration, state and local regulatory agencies. If you should have questions about legal obligations, you should consult with the U.S. Food and Drug Administration.